- Casa
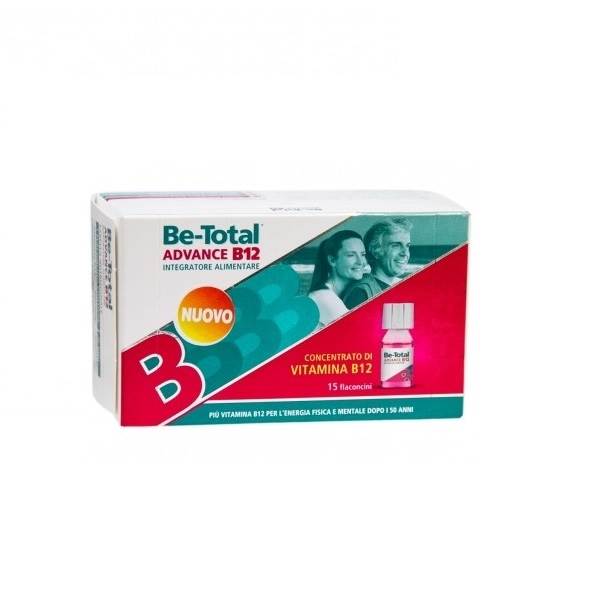
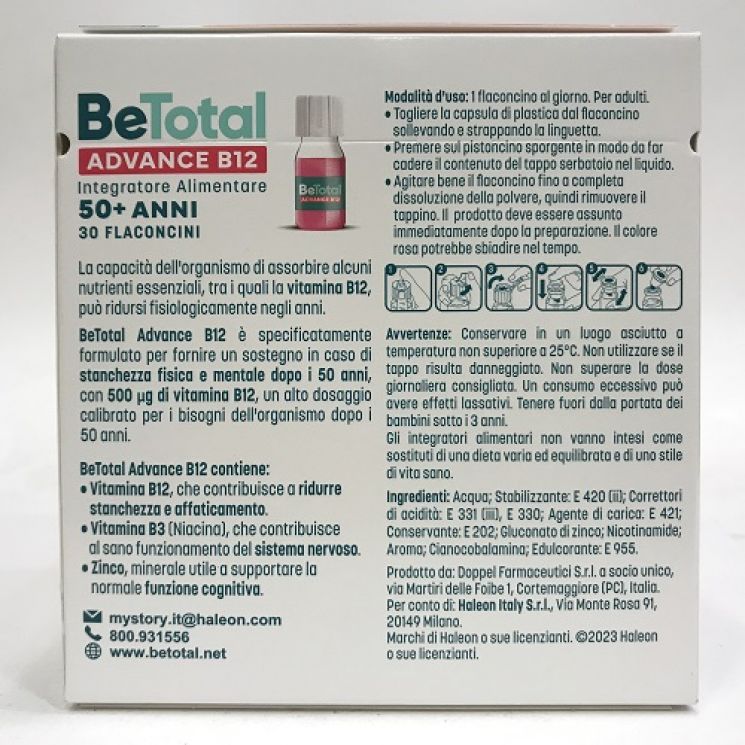
- betotal advance b12 over 50
- Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Proficiency Testing Regulations Related to Analytes and Acceptable Performance
Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Proficiency Testing Regulations Related to Analytes and Acceptable Performance
4.7 (557) · € 19.50 · In Magazzino

Definition: CLIA certified? CAP accredited? What does this mean? – Trends in Salivary Testing

Laboratory Medical Director Tabletweb Pages 1-21 - Flip PDF Download
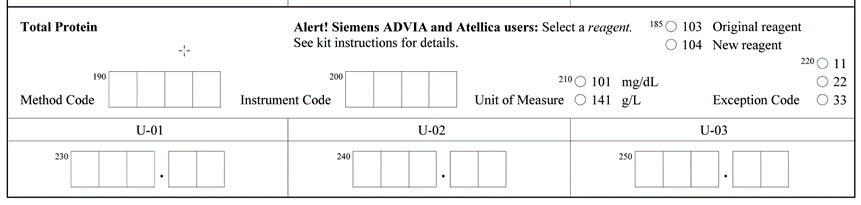
Proficiency Testing/External… College of American Pathologists

Changes to the Clinical Laboratory Improvement Amendments (CLIA)
CLIA Regulations Changing in 2024

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories

PDF) Calibration verification performance relates to proficiency testing performance

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories

Federal Register :: Clinical Laboratory Improvement Amendments of 1988 (CLIA) Fees; Histocompatibility, Personnel, and Alternative Sanctions for Certificate of Waiver Laboratories